INDICATE Newsletter October 2025
ESICM: New member of the External Expert Advisory Board

We are pleased to welcome the European Society of Intensive Care Medicine (ESICM) to the External Expert Advisory Board of the INDICATE Project!
This collaboration strengthens our shared European reach and accelerates progress toward secure, interoperable ICU data sharing, fully aligned with the ambition of the European Health Data Space (EHDS).
ESICM will be represented by Maurizio Cecconi in the External Expert Advisory Board of INDICATE.
Our first INDICATE HACKATHON
5 February 2026

Step into the future of Intensive Care innovation by participating in our Hackathon on ICU federated data usage. This dynamic event invites ICU clinicians, engineers, data scientists, and startups from the INDICATE and EIT Health network to co-create a vision for a global, real-time federated data infrastructure.
Together, we’ll explore how to securely link ICU data across national and EU systems using anonymization techniques and federated learning. While ensuring full compliance with EU regulations and the AI Act. Participants will gain hands-on experience implementing Equitable AI Models Across Diverse European ICU Populations and help us build the Value-based proposition of Federated ICU Data Usage, guided by expert mentors. Expect rich cross-pollination of ideas and help shape ethical frameworks for deploying clinical and research algorithms.
Join us for this collaborative opportunity to discuss how to better implement the ICU federated data infrastructure on Thursday 5th of February from 8:00hrs to17:00hrs in Amsterdam. The Hackathon will be a part of the 2026 ESICM Intensive Care Innovation Forum. Let’s build the future of ICU data. Secure, smart, and shared.
INDICATE Technical Lead Jan van den Brand joins EU panel on Virtual Human Twins in Brussels

The high-level event on Virtual Human Twins (VHTs), held on the 21st of October in Brussels, Belgium, brought together leading voices from the European Commission, academia, industry, and patient organisations, all united by a shared goal: accelerating innovation in health through AI, data, and trustworthy digital ecosystems.
INDICATE Design Workshop in Rotterdam at KPMG

End of September: INDICATE Design Workshop in Rotterdam at KPMG. Lots of bright minds in the room thinking about how we can advance patient-centered care and promote the use of Intensive Care data by developing and implementing responsible and trustworthy AI-models.
Interview with INDICATE co-lead
Michel van Genderen on NOS Nieuwsuur

Recently, INDICATE co-lead Michel van Genderen shared his expertise on the Dutch national news program NOS Nieuwsuur, discussing the risks associated with AI tools such as Delphi-2M, which predicts the likelihood of developing diseases.
INDICATE: What is federated data analysis?!
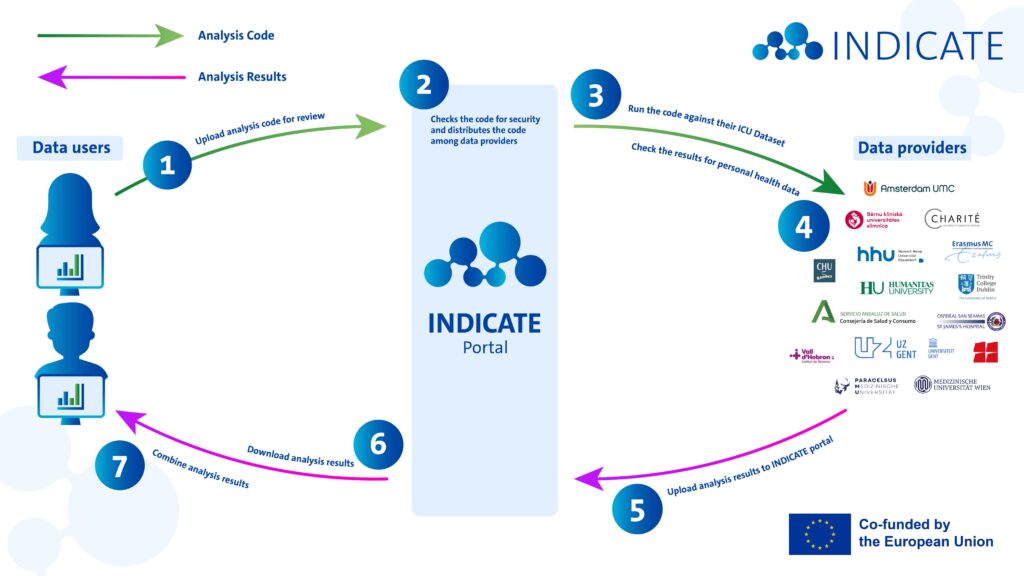
Healthcare institutions possess vast amounts of untapped data that could drive innovation and improve patient outcomes. As much as 97% of health data world-wide is not re-used. With INDICATE we aim to unlock this potential by building a federated data infrastructure for Intensive Care Units (ICUs) across Europe.
But how can you analyze data without accessing it directly? The answer lies in INDICATE’s design, specifically in its first development phase due for delivery in August 2025.
INDICATE’s federated analysis architecture enables data users, such as researchers, innovators, and policy makers, to derive insights from ICU data across multiple institutions, regions, and European countries.
This figure is conceptual, please feel free to share any feedback or suggestions!
INDICATE: What is federated data analysis?!

At the II National Congress on Innovation for the Advancement of Health Data Management and Evaluation (INNODATA 2025), held on 24–25 September 2025 in Seville (Spain), the Computational Health Informatics Group from the Virgen del Rocio University Hospital, as part of the Andalusian Health Service (Servicio Andaluz de Salud-SAS), presented their work on advancing federated integration of ICU data in Europe.


INDICATE Education Workshop
Date: November 25 – 26, 2025
Location: Athens, Greece
INDICATE Hackathon
Date: February 5, 2026
Time: 08:00 – 17.00
Location: Pakhuis de Zwijger, Amsterdam, The Netherlands

Do you have a special request? Would you like to share news or a publication? Would you like to be (digitally) connected to a certain person? Did you speak or went to an event related to INDICATE or INDICATE-subjects? Please feel very welcome to share your questions or input with: info@indicate-europe.eu
When sharing your news, please make sure to attach your photos/images or figures and always make sure when people are visible, you have their permission to use the pictures.
Below with the format you can use to share your news with us:
- What is the purpose of your news?
- What title can we use?
- Who attended (names, roles, if related to INDICATE meetings/events)?
- What were the main topics discussed or key findings?
- Were any follow-up actions or appointments agreed upon?
- Is there anything special or noteworthy?
Deadline for next newsletter submissions:
- 5 December, 2025
- 13 February, 2026
- 10 April, 2026
- 12 June, 2026
- 11 September, 2026
- 6 November, 2026

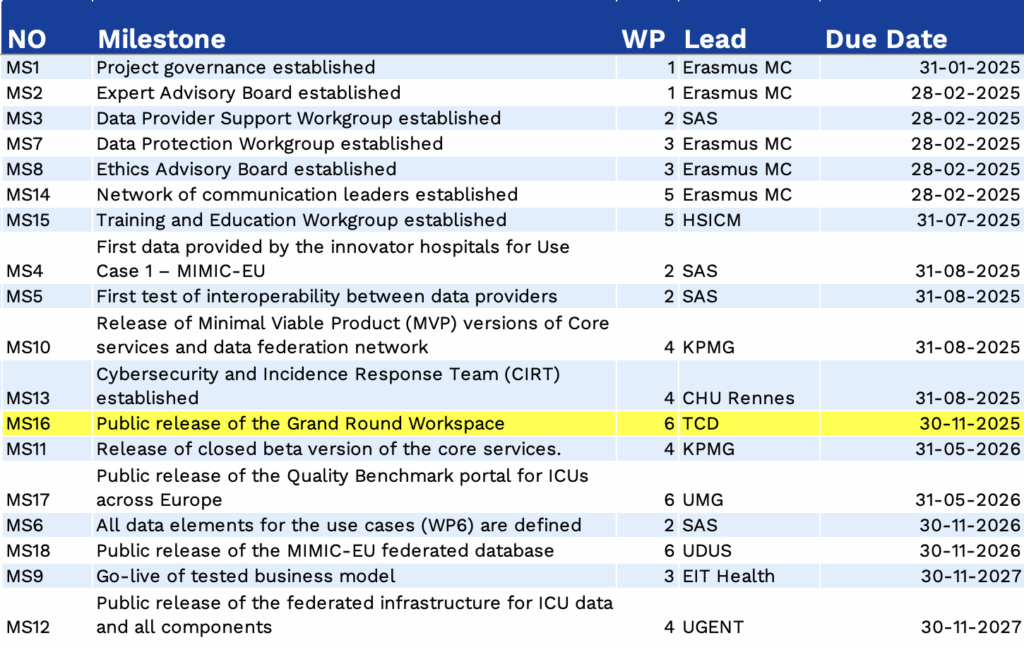
Milestones
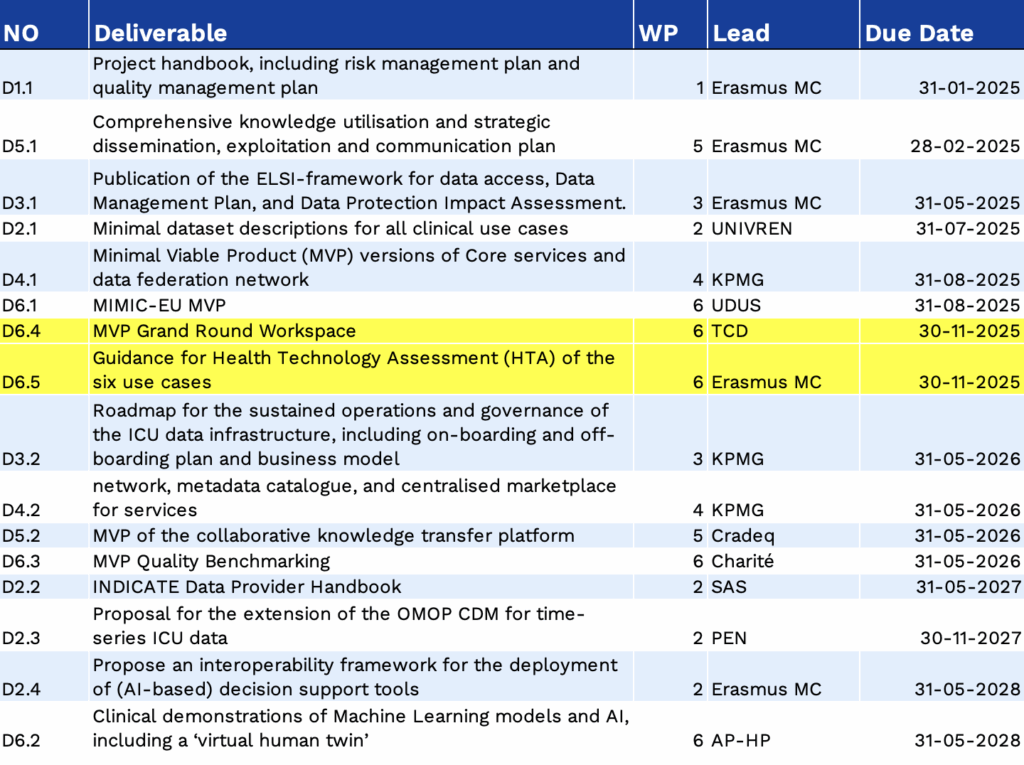
Deliverables


Michel van Genderen
Co-lead INDICATE
e: m.vangenderen@erasmusmc.nl

Christian Jung
Co-lead INDICATE
e: Christian.Jung@med.uni-duesseldorf.de

Lisanne van Prooyen Schuurman
Project Manager
e: e.vanprooyenschuurman@erasmusmc.nl

Jan van den Brand
Technical Lead
e: a.vandenbrand@erasmusmc.nl

Endorsed by:


Get in contact via e-mail:
Email address: info@indicate-europe.eu
Email address: support@indicate-europe.eu
Email address: indicate@erasmusmc.nl














































